Europe adalimumab market is expected to gain market growth in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is growing with a CAGR of 16.2% in the forecast period of 2022 to 2029 and is expected to reach USD 10,337.79 million by 2029 from USD 3,485.94 million in 2021.
The Europe Adalimumab market represents one of the most mature and strategically important segments within the region’s biologics and biosimilars ecosystem. Adalimumab, a monoclonal antibody targeting tumor necrosis factor-alpha (TNF-α), has become a cornerstone therapy for a wide range of autoimmune and inflammatory disorders. Its extensive clinical applicability, combined with expanding biosimilar adoption, continues to reshape treatment accessibility and pricing dynamics across European healthcare systems.Europe’s strong regulatory framework, universal healthcare coverage in many countries, and increasing prevalence of chronic inflammatory diseases have collectively positioned the region as a key market for both originator adalimumab and its biosimilar counterparts.
Market Overview
Adalimumab is widely prescribed for conditions such as rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, plaque psoriasis, and certain pediatric inflammatory disorders. Over the years, its clinical efficacy and safety profile have led to sustained demand across hospital pharmacies, specialty clinics, and outpatient care settings.
The European market has evolved significantly following patent expirations of the reference biologic, resulting in a rapid influx of biosimilar versions. This transition has intensified competition, reduced treatment costs, and expanded patient access, particularly in price-sensitive healthcare systems. As a result, Europe has emerged as one of the fastest-adopting regions for adalimumab biosimilars globally.
See what’s driving the Europe Adalimumab Market forward. Get the full research report:
https://www.databridgemarketresearch.com/reports/europe-adalimumab-market
Key Growth Drivers
Rising Burden of Autoimmune Diseases
The increasing prevalence of autoimmune and inflammatory diseases across Europe is a major growth catalyst. Aging populations, improved diagnostic rates, and lifestyle-related risk factors have contributed to a higher incidence of chronic conditions such as rheumatoid arthritis and inflammatory bowel disease. These disorders often require long-term biologic therapy, positioning adalimumab as a preferred treatment option.
Strong Biosimilar Adoption
Europe is widely recognized as a global leader in biosimilar acceptance. Favorable regulatory pathways, physician confidence, and payer-driven substitution policies have accelerated biosimilar penetration in the adalimumab segment. Many European countries actively encourage biosimilar prescribing through reimbursement incentives, tender systems, and national treatment guidelines.
Cost Containment and Healthcare Sustainability
European healthcare systems face increasing pressure to manage rising pharmaceutical expenditures. Adalimumab biosimilars offer a cost-effective alternative to originator products while maintaining comparable efficacy and safety. The resulting cost savings enable healthcare providers to treat a larger patient population and reallocate budgets to innovative therapies in other therapeutic areas.
Expanding Indication Approvals
Adalimumab continues to receive approvals for additional indications and patient populations, including pediatric use. This ongoing expansion broadens its clinical relevance and sustains market demand across multiple medical specialties such as rheumatology, gastroenterology, dermatology, and ophthalmology.
Market Challenges
Despite strong growth fundamentals, the Europe Adalimumab market faces several challenges that influence competitive strategies and pricing structures.
One key challenge is price erosion, particularly in countries with centralized procurement and tender-based purchasing systems. While lower prices benefit healthcare systems and patients, they can reduce profit margins for manufacturers and intensify competition.
Another challenge involves physician and patient switching concerns, especially when transitioning from an originator biologic to a biosimilar. Although regulatory authorities ensure biosimilar equivalence, some resistance remains due to brand loyalty or concerns about immunogenicity and long-term outcomes.
Additionally, market saturation in certain Western European countries has slowed volume growth, shifting competition toward pricing, service differentiation, and value-added support programs.
Segmentation Analysis
By Product Type
The market is segmented into originator adalimumab and biosimilar adalimumab products. Biosimilars now account for a growing share of total sales volume, driven by aggressive pricing strategies and payer-mandated substitution policies. Originator products, however, continue to maintain a presence in specific patient groups and markets where brand familiarity remains strong.
By Indication
Rheumatoid arthritis represents the largest indication segment, followed by inflammatory bowel diseases such as Crohn’s disease and ulcerative colitis. Dermatological indications, particularly plaque psoriasis, also contribute significantly to market demand. The broad indication profile of adalimumab reduces dependency on a single therapeutic area and enhances market stability.
By Distribution Channel
Hospital pharmacies dominate the distribution landscape due to the specialized nature of biologic therapies and the need for controlled administration and monitoring. Retail and specialty pharmacies also play an increasing role, particularly for self-administered formulations supported by patient education and home-care programs.
Regional Insights
Western Europe, including countries such as Germany, France, the United Kingdom, Italy, and Spain, constitutes the largest share of the Europe Adalimumab market. These countries benefit from advanced healthcare infrastructure, high diagnosis rates, and established biologics reimbursement frameworks.
Northern European countries demonstrate particularly strong biosimilar uptake, supported by proactive government policies and high levels of physician trust in biosimilar products. Meanwhile, Central and Eastern European markets are experiencing faster growth rates, driven by improving healthcare access, increasing government healthcare spending, and greater adoption of cost-effective biologic therapies.
Competitive Landscape
The Europe Adalimumab market is highly competitive, characterized by the presence of multiple biosimilar manufacturers alongside the originator brand. Competition is primarily driven by pricing, supply reliability, and value-added services such as patient support programs, injection devices, and real-world evidence generation.
Manufacturers are increasingly focusing on strategic partnerships with healthcare providers and payers to secure long-term supply agreements. Additionally, investments in pharmacovigilance, patient education, and digital health tools are becoming key differentiators in a crowded market environment.
Future Outlook
The future of the Europe Adalimumab market remains positive, albeit increasingly competitive. Continued biosimilar adoption will further democratize access to biologic therapies while maintaining pressure on pricing structures. Innovation in delivery systems, such as improved autoinjectors and patient-friendly formulations, is expected to enhance adherence and patient satisfaction.
Moreover, the experience gained from adalimumab biosimilars is likely to influence the adoption of future biologics and biosimilars across Europe. As healthcare systems prioritize value-based care, manufacturers that can demonstrate real-world outcomes, cost efficiency, and patient-centric solutions will be best positioned for long-term success.
Conclusion
The Europe Adalimumab market stands as a mature yet evolving segment shaped by biosimilar competition, rising chronic disease prevalence, and strong regulatory support. While pricing pressures and market saturation present challenges, the market continues to offer significant opportunities driven by expanding indications, broader patient access, and healthcare cost containment initiatives.
Browse More Reports:
Global Water Treatment Chemicals Market
Global Scented Candle Market
Global Ceramics Market
Europe Japanese Restaurant Market
Global Tuna Market
Global Japanese Restaurant Market
Global Tote Bags Market
Global Gemstones Market
Global Smart Fleet Management Market
Global Hypochlorous Acid Market
Global Toothbrush Market
Global Cataracts Market
Global Plant-Based Food Market
Global Eyewear Market
Global Processed Fruits Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- [javascript protected email address]






 rrdbmr
rrdbmr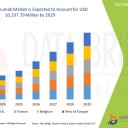
 kshdbmr
kshdbmr