
The Clinical Diagnostics Market size was valued at USD 84.49 Billion in 2024 and the total Clinical Diagnostics revenue is expected to grow at a CAGR of 6.5% from 2025 to 2032, reaching nearly USD 139.83 Billion.
Global Clinical Diagnostics Market Forecast to Reach USD 139.83 Billion by 2032The global Clinical Diagnostics Market was valued at USD 84.49 billion in 2024 and is projected to reach USD 139.83 billion by 2032, growing at a CAGR of 6.5% from 2025 to 2032. The market is witnessing robust growth due to technological advancements, rising prevalence of chronic and infectious diseases, and increasing awareness of the importance of laboratory testing in healthcare management.
Market Overview
Clinical diagnostics involves the administration of FDA-approved diagnostic tests on human biological samples, including blood, urine, and tissues. These tests provide critical insights to healthcare providers for disease detection, monitoring, and treatment evaluation. The market encompasses instruments, reagents, and other diagnostic products used in laboratories, hospitals, and point-of-care settings.
The adoption of rapid test kits, portable diagnostic instruments, and point-of-care devices has surged in recent years, enabling decentralized testing and quicker clinical decision-making. High sensitivity and specificity associated with in vitro diagnostics (IVD) are key factors driving the uptake of clinical diagnostics worldwide.
Secure your sample copy of the report today: https://www.maximizemarketresearch.com/request-sample/116258/" target="_blank"> https://www.maximizemarketresearch.com/request-sample/116258/
Market Drivers
Growing Demand for Disease Detection:
The increasing prevalence of chronic diseases such as diabetes, heart disease, and cancer, combined with the rising adoption of personalized medicine, is fueling demand for clinical diagnostic tests. Early disease detection through advanced lab tests and molecular diagnostics enables better treatment outcomes and reduces healthcare costs.
Healthcare Expenditure and Automation:
Automation in laboratory processes and retail pharmacy systems has improved efficiency, reduced medication wastage, and lowered operational costs. Advanced automated systems also enable accurate inventory management, improved patient safety, and better workflow management, boosting the adoption of clinical diagnostics.
Rise in Chronic and Infectious Diseases:
According to the WHO, noncommunicable diseases account for 72% of global deaths, highlighting the need for accurate diagnostic tools. Additionally, the COVID-19 pandemic accelerated testing demand, with initiatives such as the NIH RADx program funding the development of rapid diagnostics. Increasing prevalence of infectious diseases like HIV, hepatitis, and STIs presents further growth opportunities.
Technological Innovations:
Advancements in automated instruments, molecular diagnostic platforms, and home sample collection kits have increased accessibility, accuracy, and convenience of testing. Portable and handheld diagnostic tools are enabling point-of-care testing in remote or underserved areas.
Market Restraints
High costs of clinical diagnostic instruments and their maintenance limit adoption in certain regions. Sophisticated equipment such as RT-PCR systems can cost between USD 15,000 to over USD 90,000, and require skilled professionals, creating a barrier in developing markets.
Segment Analysis
By Test Type:
Lipid Panel Tests: Expected to grow rapidly due to rising cardiovascular disease prevalence and the role of lipid profiling in risk assessment.
Other segments include Liver Panel, Renal Panel, Complete Blood Count, Electrolyte Testing, Infectious Disease Testing, and additional diagnostic tests.
By Product:
Instruments: Dominant segment due to continuous innovations and increasing adoption in hospitals and laboratories.
Reagents: Growing demand alongside instruments for molecular and biochemical testing.
Other Products: Consumables and ancillary testing devices supporting the diagnostics workflow.
By End-User:
Hospital Laboratories: Leading segment with over 54% revenue share, driven by the availability of complex tests and higher accuracy.
Diagnostic Laboratories: Growing adoption in private labs offering specialized tests.
Point-of-Care Testing (PoC): Rapid growth fueled by demand for faster turnaround times and decentralized testing.
Other End Users: Include research institutions and academic hospitals.
Secure your sample copy of the report today: https://www.maximizemarketresearch.com/request-sample/116258/" target="_blank"> https://www.maximizemarketresearch.com/request-sample/116258/
Regional Insights
North America dominates the clinical diagnostics market, supported by a large geriatric population, high patient awareness, advanced healthcare infrastructure, and COVID-19 testing initiatives.
Europe shows steady growth due to advanced lab networks and technological innovation by companies like Siemens AG and Roche.
Asia Pacific is emerging as a key market, driven by rising healthcare investments, expanding lab infrastructure, and increasing disease prevalence in countries such as China and India.
Middle East & Africa and South America offer untapped growth potential due to improving healthcare access and infrastructure.
Key Market Players
The market is competitive, with major players focusing on innovation, strategic partnerships, and expansion:
Abbott Laboratories
Becton, Dickinson, and Company
BioMerieux
Bio-Rad Laboratories Inc.
Bioscientia Healthcare
Bio-Reference Laboratories
ARUP Laboratories
Labco S.A.
Healthscope Limited
Labcorp
Danaher Corporation
Siemens AG
Hologic Inc.
Qiagen NV
F. Hoffmann-La Roche AG
Thermo Fisher Scientific
Quest Diagnostics Inc.
Sysmex Corporation
Sonic Healthcare Ltd
Charles River Laboratories
These companies are leveraging R&D investments, product innovations, and automated platforms to expand their market presence and meet the growing demand for clinical diagnostics.
Market Outlook
The clinical diagnostics market is poised for substantial growth due to the convergence of rising chronic and infectious disease prevalence, technological advancements in diagnostic platforms, increasing patient awareness, and government funding initiatives. The increasing adoption of point-of-care testing and automated laboratory systems is expected to drive the market forward, providing critical solutions for early disease detection and effective healthcare management globally.






 supriyamaximize
supriyamaximize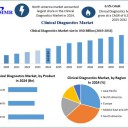
 rrdbmr
rrdbmr fefdfg
fefdfg